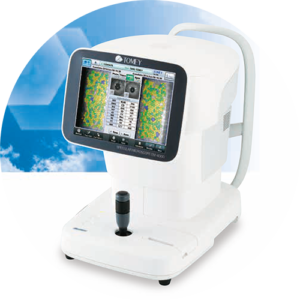
 Phoenix, Arizona – Tomey USA announces the FDA clearance of its new EM-4000 Specular Microscope. This device features the latest technology to meet all the customer´s demands. Cecil Swyers, President of Tomey USA says “the new Specular microscope has a few advantages over what is being offered by its competitors.” The new device is more accurate due to Tomey’s new Core method to count cells. All the other units use an Auto Mode that is very inaccurate, due to the fact the software misses reading the edges of the cells. The new unit has a larger screen that moves up and down to accommodate the user’s preference and a new database that can accommodate thousands of patient images and data. And a removable chip that backs up all the data on the unit. It can also output data and images to a EHR server or standalone computer. Tomey USA will exhibit the new Specular Microscope capabilities and functions at the Vision Expo East 2018 trade show in New York.
Phoenix, Arizona – Tomey USA announces the FDA clearance of its new EM-4000 Specular Microscope. This device features the latest technology to meet all the customer´s demands. Cecil Swyers, President of Tomey USA says “the new Specular microscope has a few advantages over what is being offered by its competitors.” The new device is more accurate due to Tomey’s new Core method to count cells. All the other units use an Auto Mode that is very inaccurate, due to the fact the software misses reading the edges of the cells. The new unit has a larger screen that moves up and down to accommodate the user’s preference and a new database that can accommodate thousands of patient images and data. And a removable chip that backs up all the data on the unit. It can also output data and images to a EHR server or standalone computer. Tomey USA will exhibit the new Specular Microscope capabilities and functions at the Vision Expo East 2018 trade show in New York.
About Tomey: For over 40 years, Tomey has emerged as the worldwide leader in the eye care industry. Its mission is simple; deliver the highest quality of devices that are dually cost affordable and easily operable. With its incorporation of modern touch screen technology and comprehensive software, Tomey’s entire line of ophthalmic and optometric diagnostic instruments is designed to be efficient and offer effortless user interaction.
With seasoned expertise and success in the Specular Microcope market, Tomey has managed to extend beyond and offers a line of Topographers, Auto Refractors, Auto Refractor Keratometers, and Auto Lensmeters utilizing the same initiative for optimum accuracy and operation in patient care and practice. For more information about Tomey products contact TomeyUSA, 2630 E. Mohawk Lane, Suite 136, Phoenix Arizona 85050, or by phone at 888-449-4045 visit online www.tomeyusa.com.
